Physics and Astronomy Labs/Heisenberg's uncertainty and Beethoven's fugue/gallery
Each of the three images shown above have good summaries that you can read if you click the links and scroll down a bit.
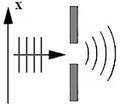
- Heisenberg microscope with wavefronts and electron scatter.svg: The electron is illuminated from below by light depicted as both photons and waves, with wavefronts shown as blue lines. Photons that enter the microscope deviate from the vertical by an angle less than ε/2, and impart momentum to the electron as they scatter off it. The depiction of the wavefronts inside the microscope is unphysical due to diffraction effects that produce a blurred image and hence uncertainty in position.
- File:Single slit diffraction simplified.png A simple image of single slit diffraction without the complication of secondary maxima - for pedagogical purposes - after viewing this image the student should see the actual pattern at
- File:HAtomOrbitals.png is what the fuss is all about. Because of quantum mechanics and uncertainty, the electron is best represented as a cloud around the atom. Shown are the energy wavefunction amplitudes for hydrogen. Brightness is a measure of probability that the electron is at that location. [1]